veriseq nipt v2
The automated in-lab IVD solution will enable NGG Thailand to introduce the Qualifi Prenatal Test. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
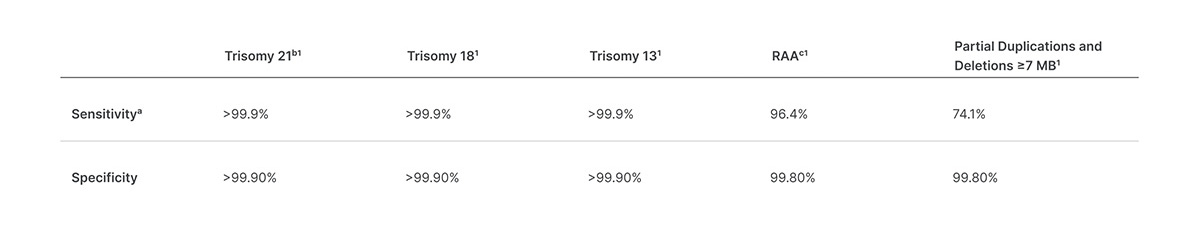
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Welcome to Immense Discovery Power.
. VeriSeq NIPT v2 offers superior performance to any IVD noninvasive prenatal testing solution available and genome-wide coverage for in-lab offerings. The assay provides information about fetal chromosomal status as early as 10. There are 2 screening options.
EM0051 The Batch ID is greater than 26 characters in length. Skip to content ProductsLearnCompanySupportRecommended Links Products Instruments Kits Reagents Selection Tools Software Analysis Services Popular Products Explore All Products Instruments. Set up the run as a dual index paired-end 151-cycle sequencing run.
NovaSeq 6000 Sequencing System is by far our most powerful instrument designed to adapt to your needs. Basic and genome wide. On average 10 of the cfDNA circulating in maternal blood is from the fetus.
Watch the video to find out why laboratories In Europe have implemented VeriSeq NIPT1. VeriSeq NIPT Solution v2 Package Insert 1000000078751 v06 PDF 1 MB Aug 16 2021. All Reproductive Health Products.
NextSeq 10002000 Reagents. This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome. Circulating cell-free DNA cfDNA from both the fetus and the mother is found in maternal blood.
When running the NextSeq in Standalone mode enter the following parameters on the Run Setup Screen. Test VeriSeq NIPT Solution v2 používá celogenomové sekvenování k detekci částečných. Basic screening provides aneuploidy status information for chromosomes 21 18 13 X.
VeriSeq NIPT Solution v2 Package Insert Translated into. VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and deletions for all autosomes and. Documentation product files FAQs and other support resources for the VeriSeq NIPT Solution v2 VeriSeq NIPT Solution v2 Products Learn Company Support Recommended Links.
VeriSeq NIPT Solution v2 je diagnostický test in vitro který je určen k použití jako vyšetřovací test zjišťující celogenomové genetické anomálie plodu ze vzorků periferní plné krve žen které jsou alespoň 10 týdnů těhotné. Why did they start to think about onboarding NIPT technology2. RevisionHistory Document Date DescriptionofChange Document 1000000067940v06 August 2021 UpdatedEUAuthorizedRepresentativeaddress.
P1 reagents are now available for NextSeq 1000NextSeq 2000 Systems offering added flexibility to meet your projects needs. Réactif VeriSeq NIPT Solution v2 - Illumina INFORMATION AUX UTILISATEURS - Dispositifs médicaux de diagnostic in vitro - PUBLIÉ LE 30062022 A A-Cette action de sécurité est enregistrée à lANSM sous le n R2201974. Đổi tên đợt thành tên không chứa ký tự văn bản đặc biệt nào.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing. NIPT brochure for Verseq2 V11 Claria Non-Invasive Prenatal Test NIPT Provide the best clarity and reassurance to your patients Methodology. The CE-IVD VeriSeq NIPT Solution v2 is now registered for use in Thailand Vietnam Singapore South Korea Australia New Zealand Israel.
En accord avec ANSM la société Illumina a informé les utilisateurs du réactif VeriSeq NIPT Solution v2 de sa position concernant lutilisation. This enables the VeriSeq NIPT Solution v2 to detect anomalies that targeted assays miss and deliver more insights into the health of a pregnancy. Expanded noninvasive prenatal testing looking beyond trisomies T21 T18 and T13.
VeriSeq NIPT Solution v2 is an integrated platform that uses paired-end whole-genome sequencing to detect fetal anomalies. ID đợt dài hơn 26 ký tự. An efficient 3-step workflow allows.
The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for. Instructions for using the VeriSeq NIPT Solution v2. Extensive validation of the VeriSeq NIPT Solution v2 confirmed high concordance with clinical reference data and a low test failure rate.
PDF 1 MB Aug 13 2021. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. The VeriSeq NIPT Solution v2 is an in vitro diagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation.
Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor. VeriSeq NIPT Solution v2 is a CE-IVD approved and next-generation sequencing NGS-based method to noninvasive prenatal testing NIPT. VeriSeq NIPT Solution v2 chỉ chấp nhận số chữ cái dấu gạch dưới và dấu gạch ngang cho tất cả các trường dữ liệu.
Sequencing with the VeriSeq NIPT Solution v2 enables comprehensive insights reducing the need for invasive tests. At Illumina our goal is to apply innovative technologies to the analysis of genetic variation and function making studies possible that were not even imaginable just a few years ago. FASTQ files streamed into BaseSpace can be analyzed using the BWA Enrichment App or the Issac Enrichment App v20 and v21 custom manifest workflow.
VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada.

Illumina Twitter પર Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
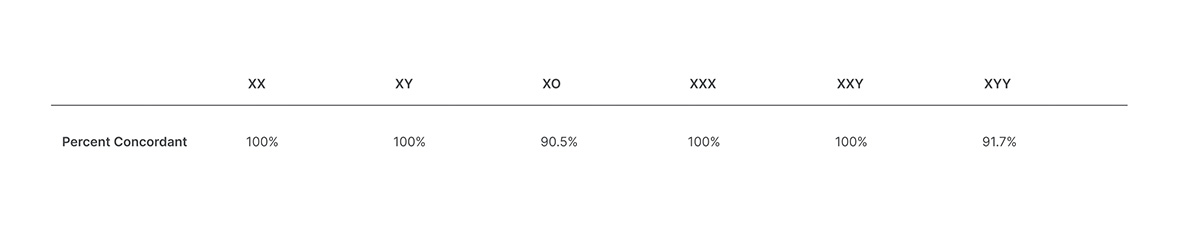
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Analysis Of Cfdna Using A Modified Illumina Veriseq Non Invasive Download Scientific Diagram
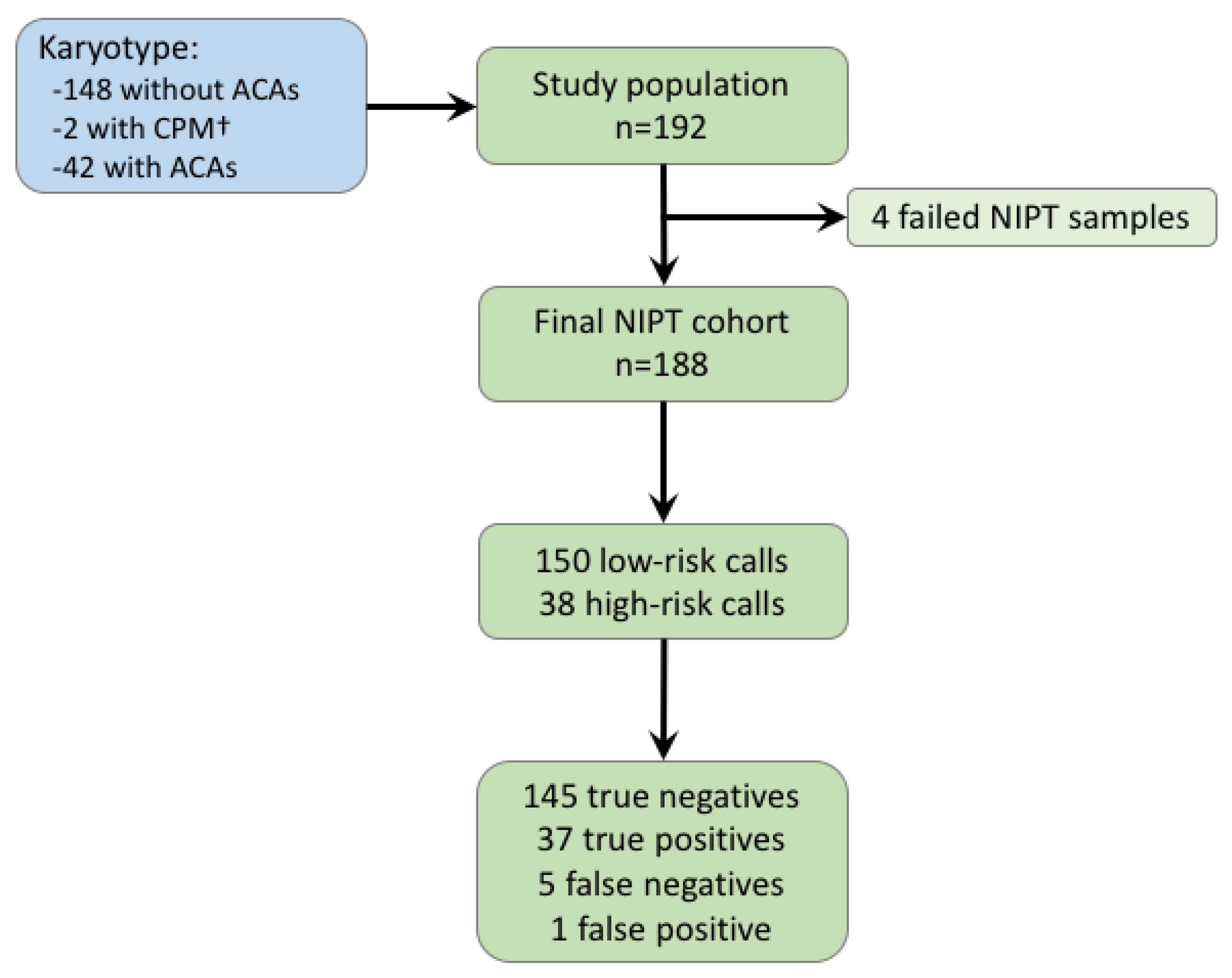
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

The Veriseq Nipt Solution Youtube
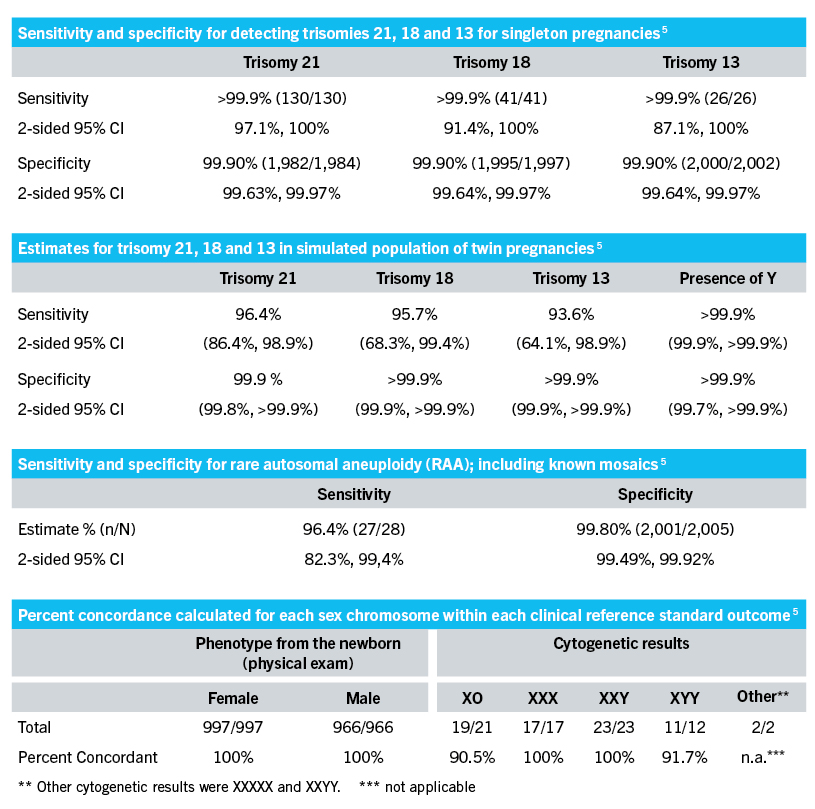
Performance Qualification Praenatest

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Genetica

Veriseq Nipt Solution V2 Support

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab



Comments
Post a Comment